Nanomanufacturing involves scaled-up, reliable, and cost-effective manufacturing of nanoscale materials, structures, devices, and systems. It also includes research, development, and integration of top-down processes and increasingly complex bottom-up or self-assembly processes. In more simple terms, nanomanufacturing leads to the production of improved materials and new products.
Higher Performance Nanomaterials from Wood
| The U.S. Department of Agriculture, Forest Service Forest Products Laboratory (FPL) unveiled its Nanocellulose Pilot Plant at a ceremony in July, 2012. The FPL started the pilot plant primarily because cellulose nanomaterials were not available commercially or from standard chemical supply companies; the pilot plant is intended to supply quantities sufficient for development of applications. The University of Maine’s Nanofiber Pilot Plant was constructed at the same time and also provides samples for application development. The Forest Service sees the commercialization of cellulose nanomaterials as a powerful tool to increase manufacturing opportunities and high paying jobs in rural America. Nanocellulose materials are comparable to high performance synthetic fibers in tensile strength but are derived from America’s renewable and sustainable wood-based sources. Eventual costs for |  UMaine’s Nanofiber Pilot Plant, photo courtesy of UMaine Process Development Center UMaine’s Nanofiber Pilot Plant, photo courtesy of UMaine Process Development Center |
large-scale commercial production aim to be considerably lower than currently available high performance synthetic fibers and only slightly greater than e-glass. Cellulose nanomaterials also have unique photonic and piezoelectric properties. Potential applications include use in structural components, building materials, high-strength paper and packaging, sensors, and biodegradable electronics. Additionally, cellulose nanomaterials could be used as fiber reinforcement in composites requiring optical clarity as well as high strength.
Powerful Tool for Moving Nanotechnology into Clinical Applications
Currently, most nanoparticles used in biomedical research studies are made in small batches using labor-intensive and time-consuming methods. While small batches are adequate for early studies, this limits the translation of promising technologies to pre-clinical studies and clinical applications, where orders of magnitude more material are needed. With support from the National Heart, Lung, and Blood Institute Programs of Excellence in Nanotechnology, the Langer laboratory at MIT developed a method for the production of gram quantities per hour of highly reproducible lipid polymer nanoparticles. This method is a powerful tool for moving nanotechnology towards clinical application. Drugs and other therapeutics can be readily incorporated into these nanoparticles, and the technology can facilitate good manufacturing practices for production and clinical evaluation. Researchers are using this method to manufacture a broad range of nanoparticles with different therapeutic payloads that are being investigated for the treatment of cardiovascular diseases. Promising candidates can be immediately moved into large animal models since the technology allows for the production of larger quantities as needed.
Reference Materials
| Reference materials ensure fair comparisons and confidence in measurements that underpin commerce and trade. These materials, which are measured in a controlled manner using trusted methods, are mainly used for instrument calibration, method development and validation, and interlaboratory testing, all of which are essential to ensure accurate and reproducible measurements. The Department of Commerce’s National Institute of Standards and Technology (NIST), the Nation’s measurement institute, is a principal producer of reference materials used on a daily basis by scientists and engineers in industry, government, and academia. In response to stakeholder needs, NIST has produced ten nanoscale reference materials through 2015 including nanoparticles of gold, silver, titanium dioxide, silicon, and polystyrene, as well as carbon nanotubes. These reference materials have been purchased by national and international organizations for a host of research and development activities. For example, over 1,000 | 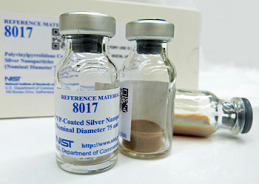 NIST's silver nanoparticle reference material is designed for extended shelf life to support environmental health and safety studies. Photo courtesy of Hackley/NIST NIST's silver nanoparticle reference material is designed for extended shelf life to support environmental health and safety studies. Photo courtesy of Hackley/NIST |
units of the gold nanoparticle reference materials, developed in cooperation with the National Cancer Institute, have been sold not only for biomedical applications—such as nanomaterial-based cancer drugs—but also for other applications that enable the development, manufacture, and risk assessment of nanotechnology-enabled consumer products.


